How animals get their skin patterns is a matter of physics
New research clarifying how could improve medical diagnostics and synthetic materials.

This article was originally featured on The Conversation.
Patterns on animal skin, such as zebra stripes and poison frog color patches, serve various biological functions, including temperature regulation, camouflage and warning signals. The colors making up these patterns must be distinct and well separated to be effective. For instance, as a warning signal, distinct colors make them clearly visible to other animals. And as camouflage, well-separated colors allow animals to better blend into their surroundings.
In our newly published research in Science Advances, my student Ben Alessio and I propose a potential mechanism explaining how these distinctive patterns form–that could potentially be applied to medical diagnostics and synthetic materials.
A thought experiment can help visualize the challenge of achieving distinctive color patterns. Imagine gently adding a drop of blue and red dye to a cup of water. The drops will slowly disperse throughout the water due to the process of diffusion, where molecules move from an area of higher concentration to lower concentration. Eventually, the water will have an even concentration of blue and red dyes and become purple. Thus, diffusion tends to create color uniformity.
A question naturally arises: How can distinct color patterns form in the presence of diffusion?
Movement and boundaries
Mathematician Alan Turing first addressed this question in his seminal 1952 paper, “The Chemical Basis of Morphogenesis.” Turing showed that under appropriate conditions, the chemical reactions involved in producing color can interact with each other in a way that counteracts diffusion. This makes it possible for colors to self-organize and create interconnected regions with different colors, forming what are now called Turing patterns.
However, in mathematical models, the boundaries between color regions are fuzzy due to diffusion. This is unlike in nature, where boundaries are often sharp and colors are well separated.
Our team thought a clue to figuring out how animals create distinctive color patterns could be found in lab experiments on micron-sized particles, such as the cells involved in producing the colors of an animal’s skin. My work and work from other labs found that micron-sized particles form banded structures when placed between a region with a high concentration of other dissolved solutes and a region with a low concentration of other dissolved solutes.
In the context of our thought experiment, changes in the concentration of blue and red dyes in water can propel other particles in the liquid to move in certain directions. As the red dye moves into an area where it is at a lower concentration, nearby particles will be carried along with it. This phenomenon is called diffusiophoresis.
You benefit from diffusiophoresis whenever you do your laundry: Dirt particles move away from your clothing as soap molecules diffuse out from your shirt and into the water.
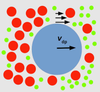
Drawing sharp boundaries
We wondered whether Turing patterns composed of regions of concentration differences could also move micron-sized particles. If so, would the resulting patterns from these particles be sharp and not fuzzy?
To answer this question, we conducted computer simulations of Turing patterns–including hexagons, stripes and double spots–and found that diffusiophoresis makes the resulting patterns significantly more distinctive in all cases. These diffusiophoresis simulations were able to replicate the intricate patterns on the skin of the ornate boxfish and jewel moray eel, which isn’t possible through Turing’s theory alone.
Further supporting our hypothesis, our model was able to reproduce the findings of a lab study on how the bacterium E. coli moves molecular cargo within themselves. Diffusiophoresis resulted in sharper movement patterns, confirming its role as a physical mechanism behind biological pattern formation.
Because the cells that produce the pigments that make up the colors of an animal’s skin are also micron-sized, our findings suggest that diffusiophoresis may play a key role in creating distinctive color patterns more broadly in nature.
Learning nature’s trick
Understanding how nature programs specific functions can help researchers design synthetic systems that perform similar tasks.
Lab experiments have shown that scientists can use diffusiophoresis to create membraneless water filters and low-cost drug development tools.
Our work suggests that combining the conditions that form Turing patterns with diffusiophoresis could also form the basis of artificial skin patches. Just like adaptive skin patterns in animals, when Turing patterns change–say from hexagons to stripes–this indicates underlying differences in chemical concentrations inside or outside the body.
Skin patches that can sense these changes could diagnose medical conditions and monitor a patient’s health by detecting changes in biochemical markers. These skin patches could also sense changes in the concentration of harmful chemicals in the environment.
The work ahead
Our simulations exclusively focused on spherical particles, while the cells that create pigments in skin come in varying shapes. The effect of shape on the formation of intricate patterns remains unclear.
Furthermore, pigment cells move in a complicated biological environment. More research is needed to understand how that environment inhibits motion and potentially freezes patterns in place.
Besides animal skin patterns, Turing patterns are also crucial to other processes such as embryonic development and tumor formation. Our work suggests that diffusiophoresis may play an underappreciated but important role in these natural processes.
Studying how biological patterns form will help researchers move one step closer to mimicking their functions in the lab–an age-old endeavor that could benefit society.
Ankur Gupta is the Assistant Professor of Chemical and Biological Engineering at the University of Colorado, Boulder. Disclosure statement: Ankur Gupta receives funding from NSF (CBET – 2238412) and ACS Petroleum Research Fund (65836 – DNI9).